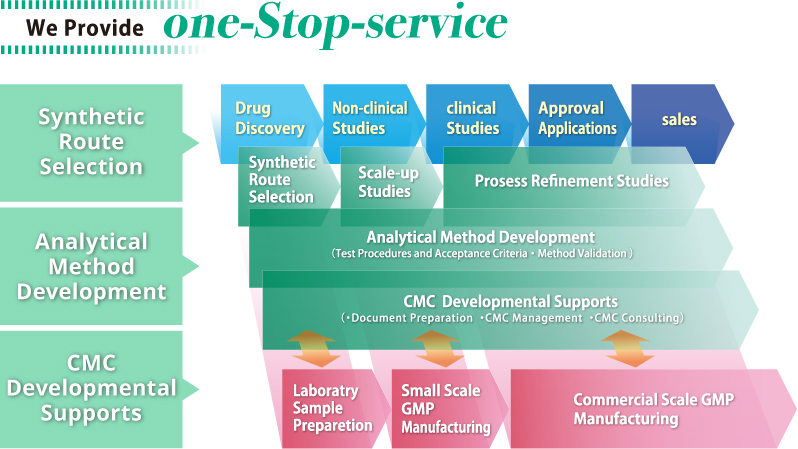
CMC Solution Services


We provide “CMC solution service” (Sample synthesis, Analytical test, Development of
synthetic procedure, Development of analytical procedure, and CMC developmental
support) as One-Stop Service at all Research and Developmental stages.
Development of Synthetic Procedure


We meet the various customer's needs by offering the service of “API process development and Quality design” based on our experiences and know-how accumulated in contract manufacturing for API or intermediates so far. Experts of the process development provide CMC solution services seamlessly and speedily from synthetic procedure studies including synthetic route scouting to API manufacturing.
- Synthesis of API/intermediate samples for various tests
- Synthetic route scouting based on reaction safety, production cost, etc. (SELECT criteria)
- Extraction of issues that may occur in actual manufacturing and confirmation of tolerances. (PAR study)
- Synthesis and identification for impurities, metabolites, decomposition product, standard reference material, etc.
- Investigate the causes of problems identified in actual manufacturing and propose improvements.
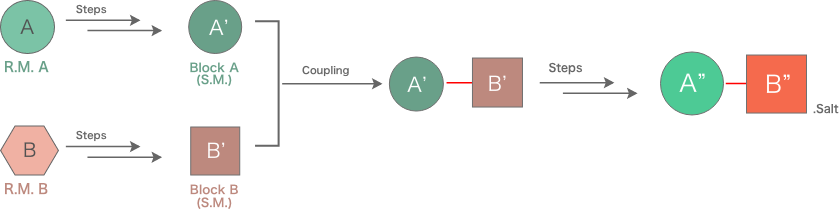
- Synthetic route scouting for API and intermediates (Major pharmaceutical company)
- The drastic improvement of API manufacturing process (Major pharmaceutical company, Bio-venture)
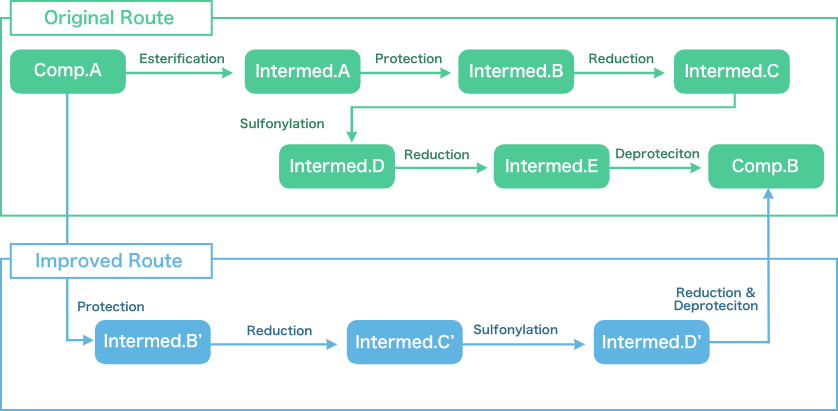
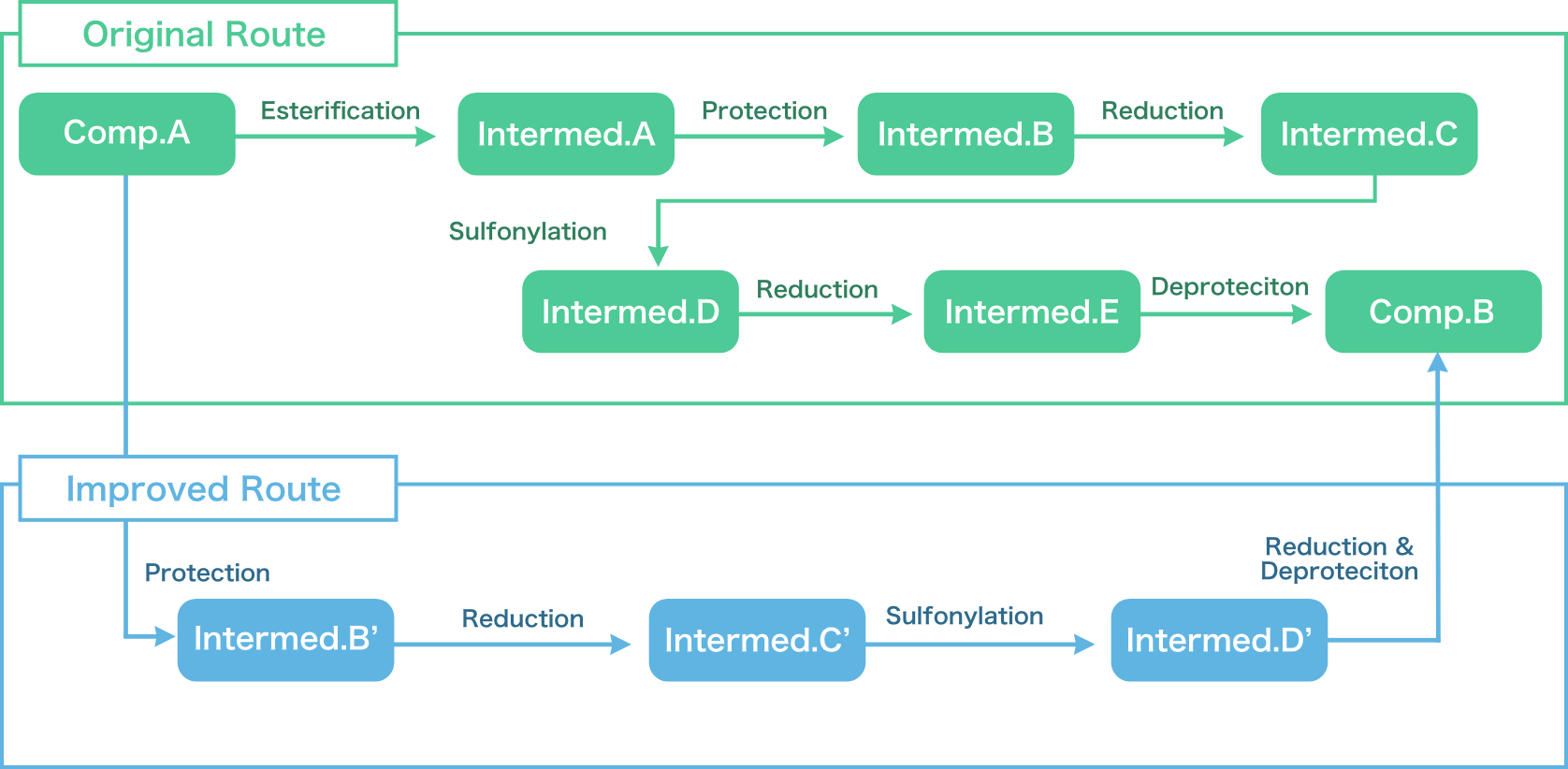
| Items | Original Route | Improved Route |
|---|---|---|
| Steps | 6 Steps | 4 Steps |
| Overall Yield | 70% | 70% |
| Challenge |
|
|
| Items | Original Method | Improved Method |
|---|---|---|
| Clinical Stage | Phase 1 | Phase 2 |
| Steps | 8 Steps | 8 Steps |
| Challenge | Early development level manufacturing methods
|
Manufacturing methods for commercial production
|
Development of Analytical Procedure


Experts of CMC development meet the various supports of customer’s quality issues at every stage of drug development. We can develop analytical methods that can evaluate the quality of drug substance. Thanks to our internal close relationship, collaboration and communication among R&D and manufacturing members we can surely grasp impurity management strategies of drug substance and intermediate. Furthermore, we can conduct various analytical tests based on both quality standards of GMP and regulations for quality.
- Analytical method scouting
- Development of test methods (including validation of analytical methods) and setting of standards for physical and chemical tests, microbiological tests, evaluation of physical properties, related substances, etc. according to the development stage.
- Development of high-sensitivity analytical method (including validation of analytical methods)
- Development of elemental impurities analytical method using ICP-MS (including validation of analytical methods)
- Proposal of quality control strategy for APIs according to development stage (for major pharmaceutical manufacturer)
- Analytical method development and method validation for investigational new drugs, intermediates, starting materials and in-process control test (for major pharmaceutical manufacturers & drug discovery bio-ventures)
- Setting of specifications and test methods for APIs according to the development stage (for a drug discovery bio-venture)
- Data acquisition for pharmaceutical manufacturing and marketing approval application (for a major pharmaceutical manufacturer)
- Preparation of IND application materials and investigational new drug summaries (for a drug discovery biotech company)
- Support for responding to inquiries from regulatory authorities (for a major pharmaceutical manufacturer)
CMC Developmental Supports


Experts of CMC development meet the various customer's needs from early development stage to new drug application. We provide long-term process management strategy (various regulation correspondence, construction of the supply chain, new drug application, commercial manufacturing) through the whole drug life cycle as "One-Stop" service by fully utilizing our production facilities.
- Propose CMC development strategies according to the drug development stage
- Obtaining data for application using PAT (Process Analytical Technology) tools
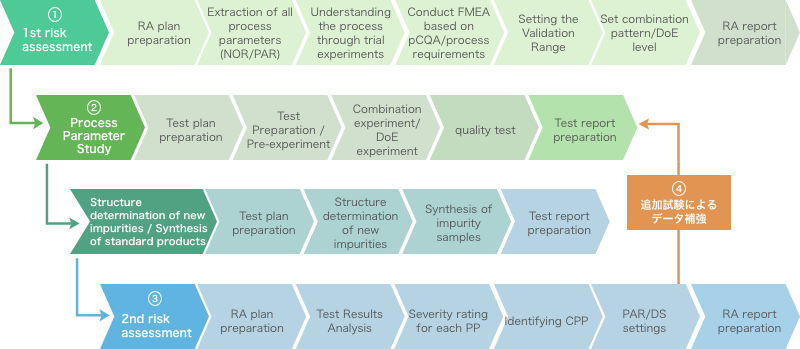
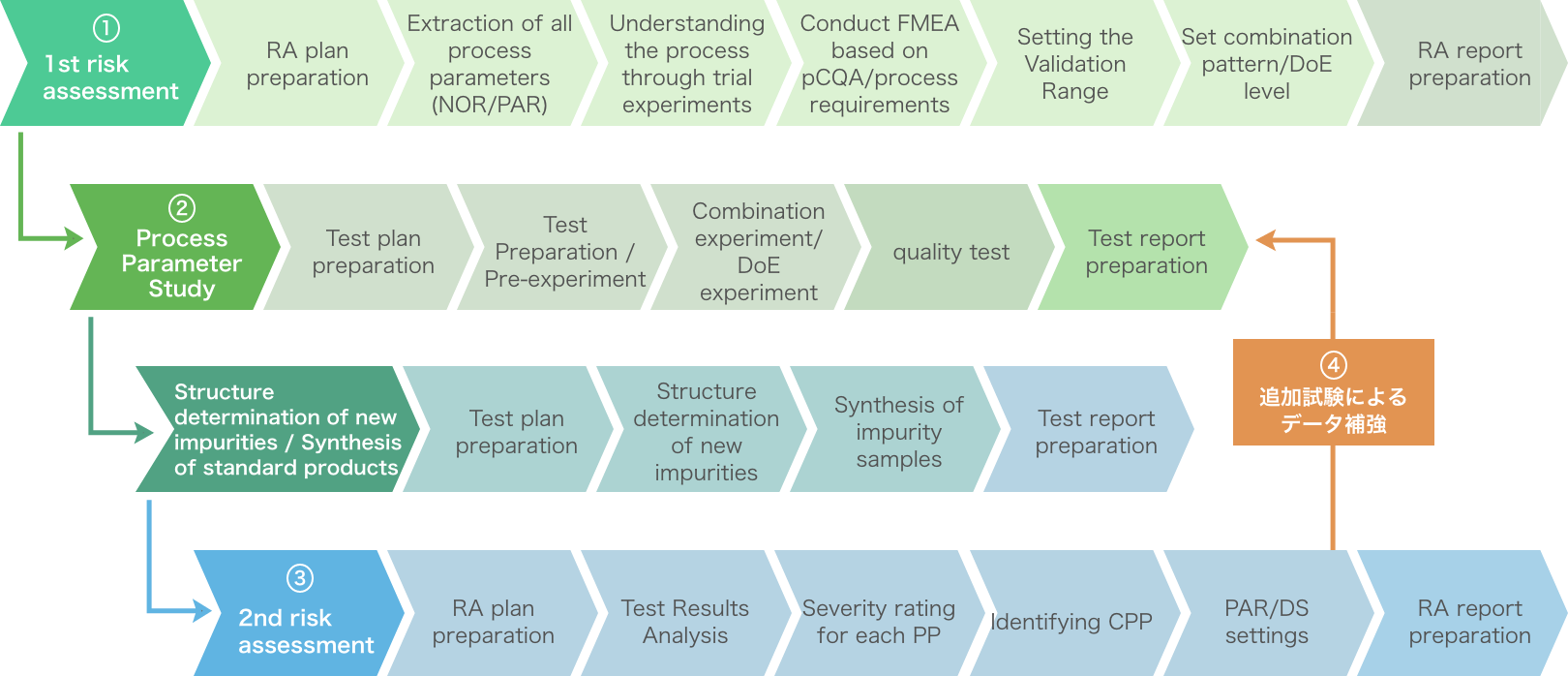
- Conduct risk assessment (FMEA) for QbD application, and obtain Design Space using DoE and provide the results.
- Preparation and documentation for IND, NDA and Drug Master File, official inquiry correspondence
- CMC developmental supports by outsourcing (e.g. polymorph screening, in silico screening (ICH M7), synthesis of radio-labeled compounds, MSDS authoring service, CMC consulting, intellectual property investigations, etc.), and project management
- Management of raw material pharmaceutical suppliers compatible with PIC/S GMP and construction of the supply chain
- Process parameter studies for application using the PAT equipment (Major pharmaceutical company)
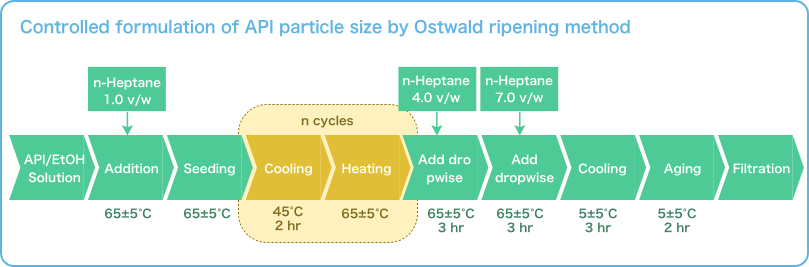
Repeated Ostwald ripening and crystallization trends
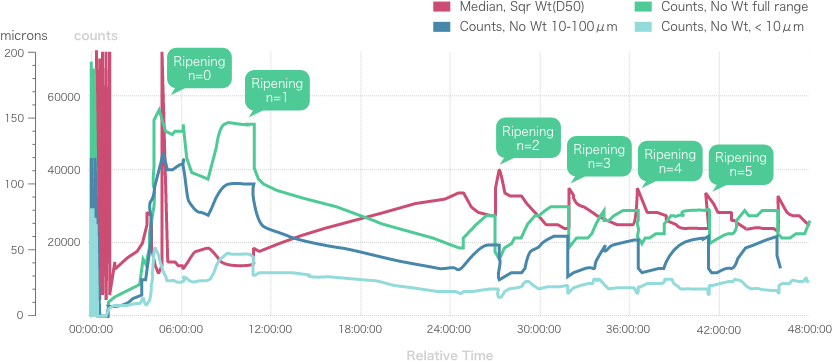
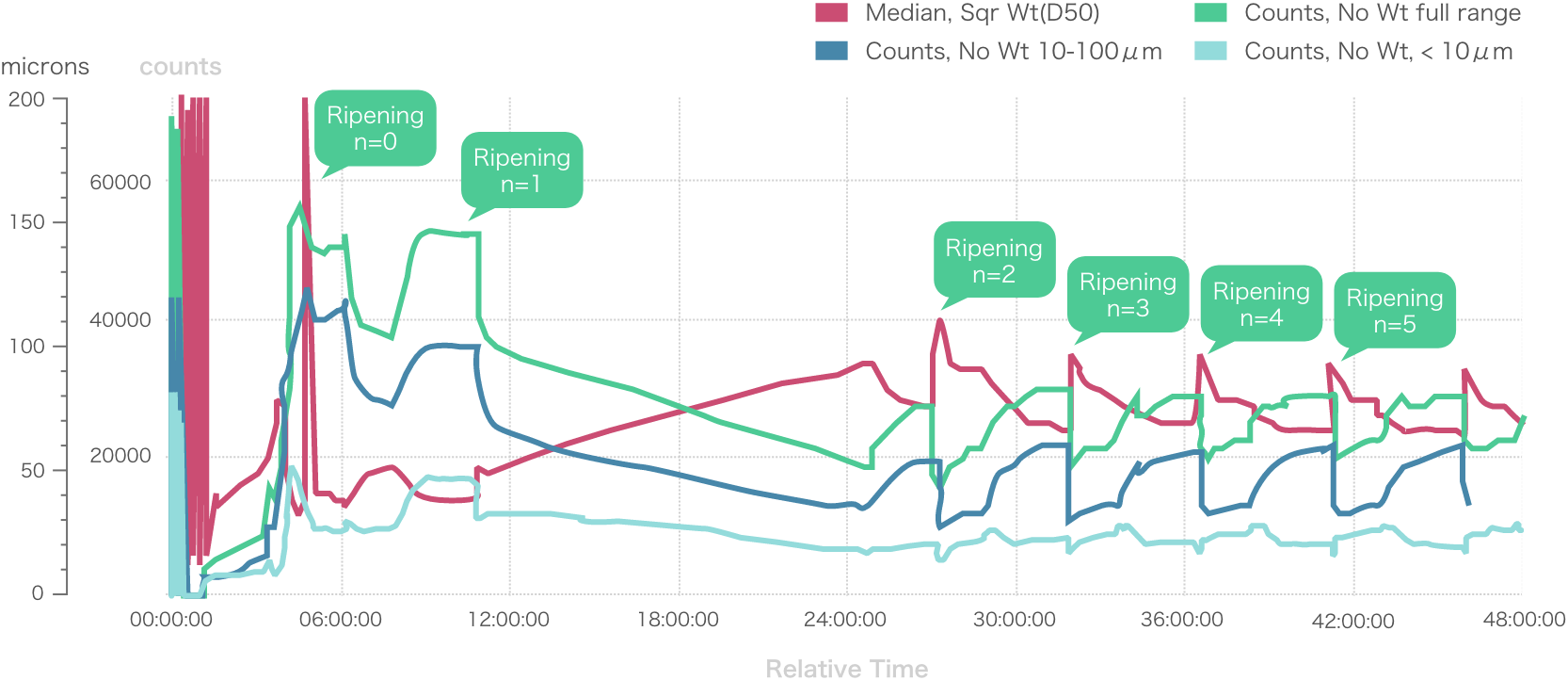
Repeated Ostwald ripening and crystallization trends
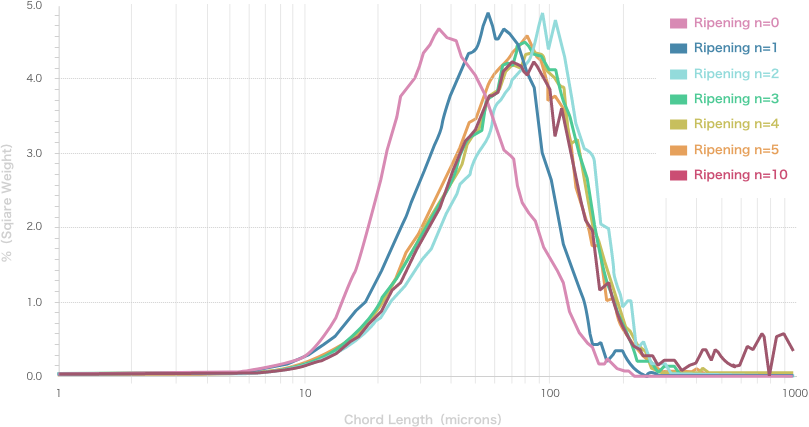
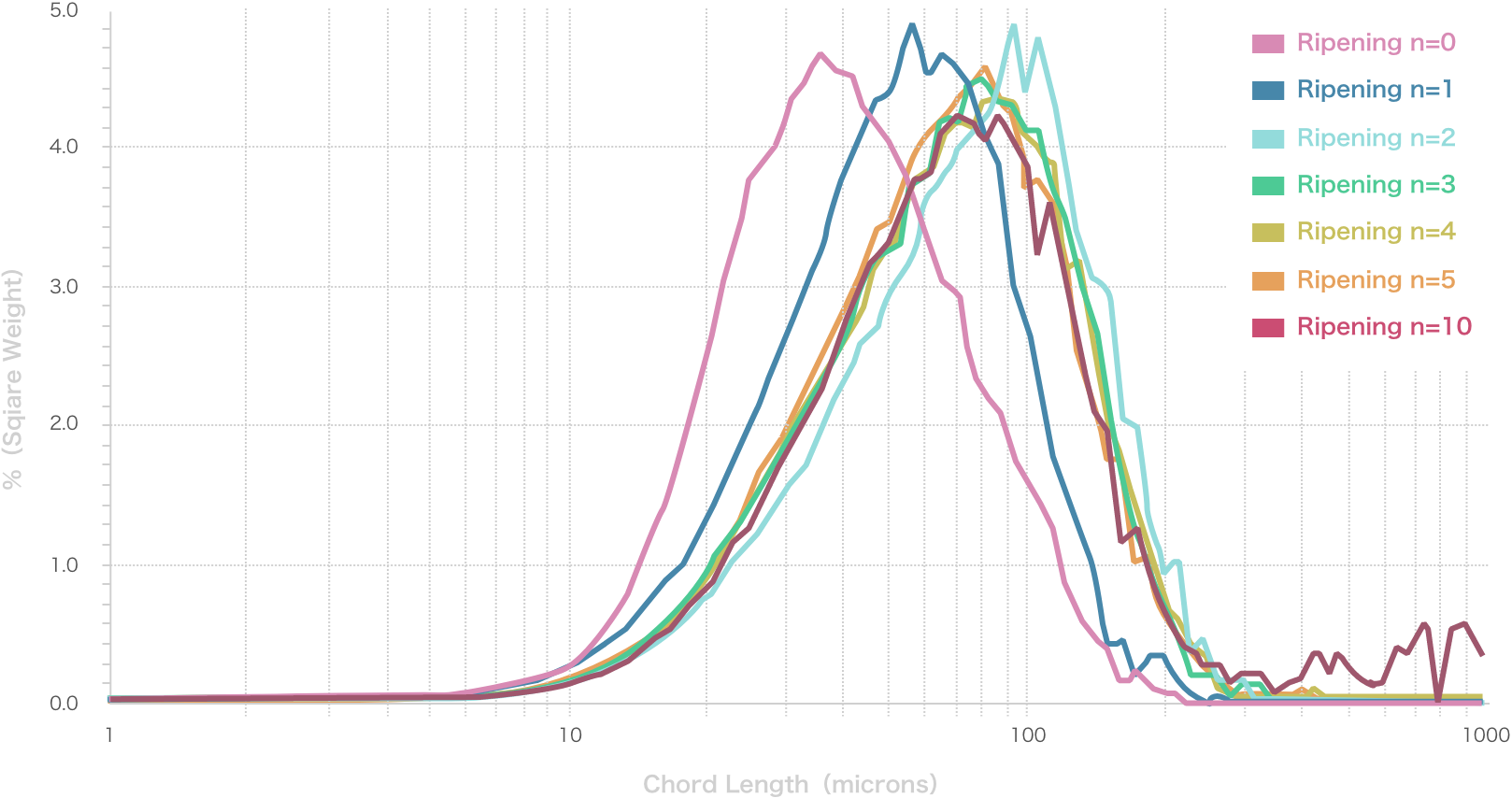
Repetition of Ostwald ripening and transition of crystal appearance
Ripening n=0
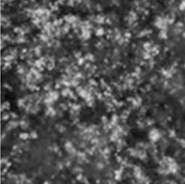
Ripening n=1

Ripening n=2
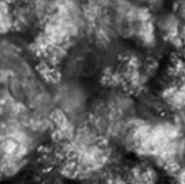
Ripening n=3
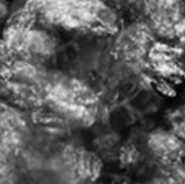
Ripening n=4

Ripening n=5
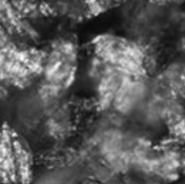
Ripening n=10

Repeated Ostwald ripening promotes crystal growth to the desired grain size.
Image of obtaining lab data for approval application (CPP identification and PAR/design space construction)
| Process Operation | Process Parameters | Significance |
|---|---|---|
| Reaction | Reagent [eq.] | Critical |
| Solvent [vol/wt] | Not critical | |
| Temperature [°C] | Critical | |
| Pressure [MPa] | Not critical | |
| Agitation [rpm] | Not critical | |
| Work up | Extraction solvent [vol/wt] | Not critical |
| End point of Concentration [vol/wt] | Critical | |
| Temperature [°C] | Not critical | |
| And more... |