Oligonucleotide Synthesis/Analysis
Oligonucleotide
Synthesis/Analysis




In recent years, middle-sized molecular medicines, including nucleic acids, peptides, and antibodies, have gained significant attention. Among these, nucleic acid medicines synthesized through chemical methods, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), have experienced a rapid increase in approvals in recent years. Capitalizing on the expertise we have gained by working with small molecules, Juzen Chemical launched our nucleic acid CDMO business in 2022.
Sample Synthesis – Process Development
We support all stages from laboratory sample synthesis to manufacturing process development and GMP manufacturing.
Drawing on the expertise we have amassed in process development and GMP manufacturing of small molecules, we offer products and solutions specifically tailored to meet our customers' various development stages.
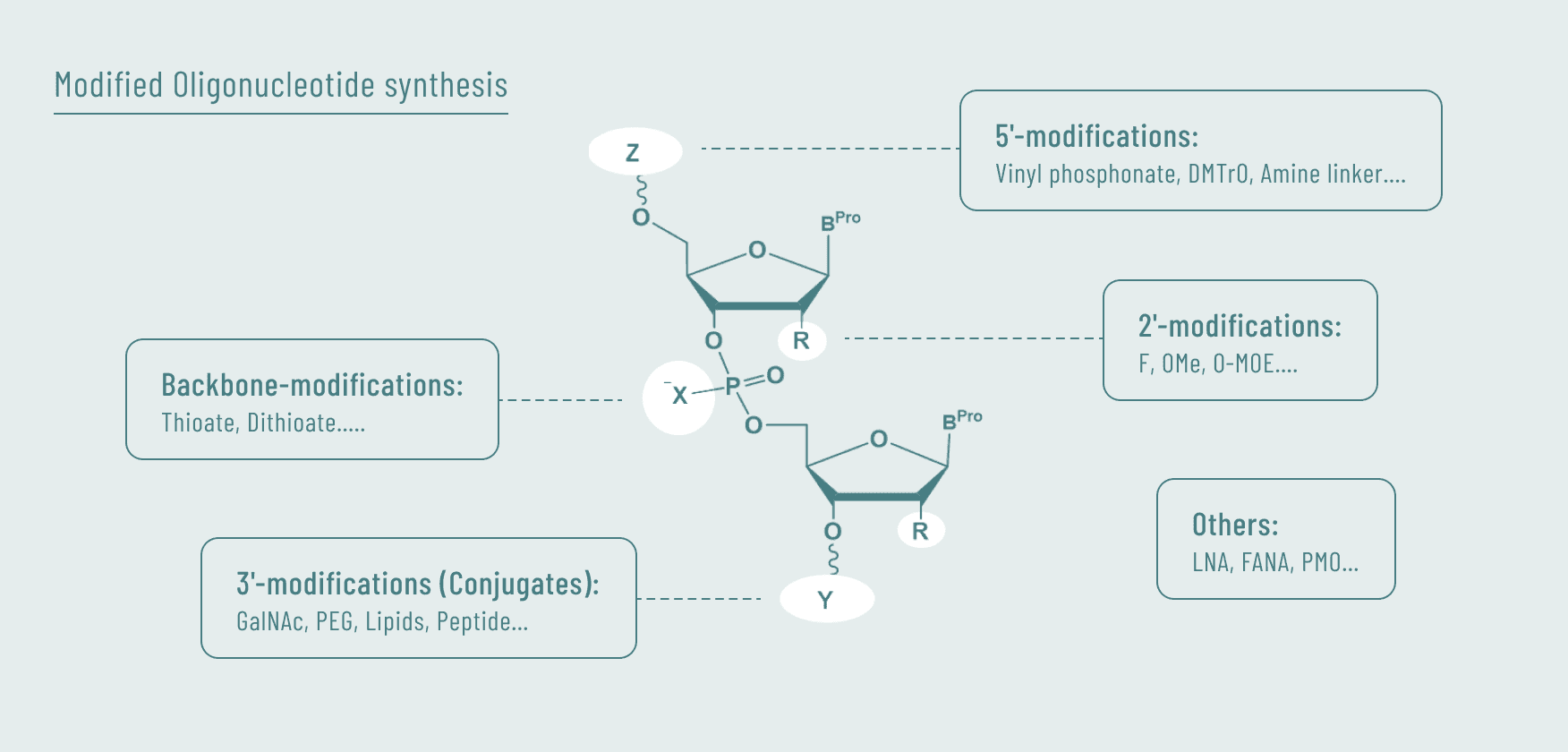
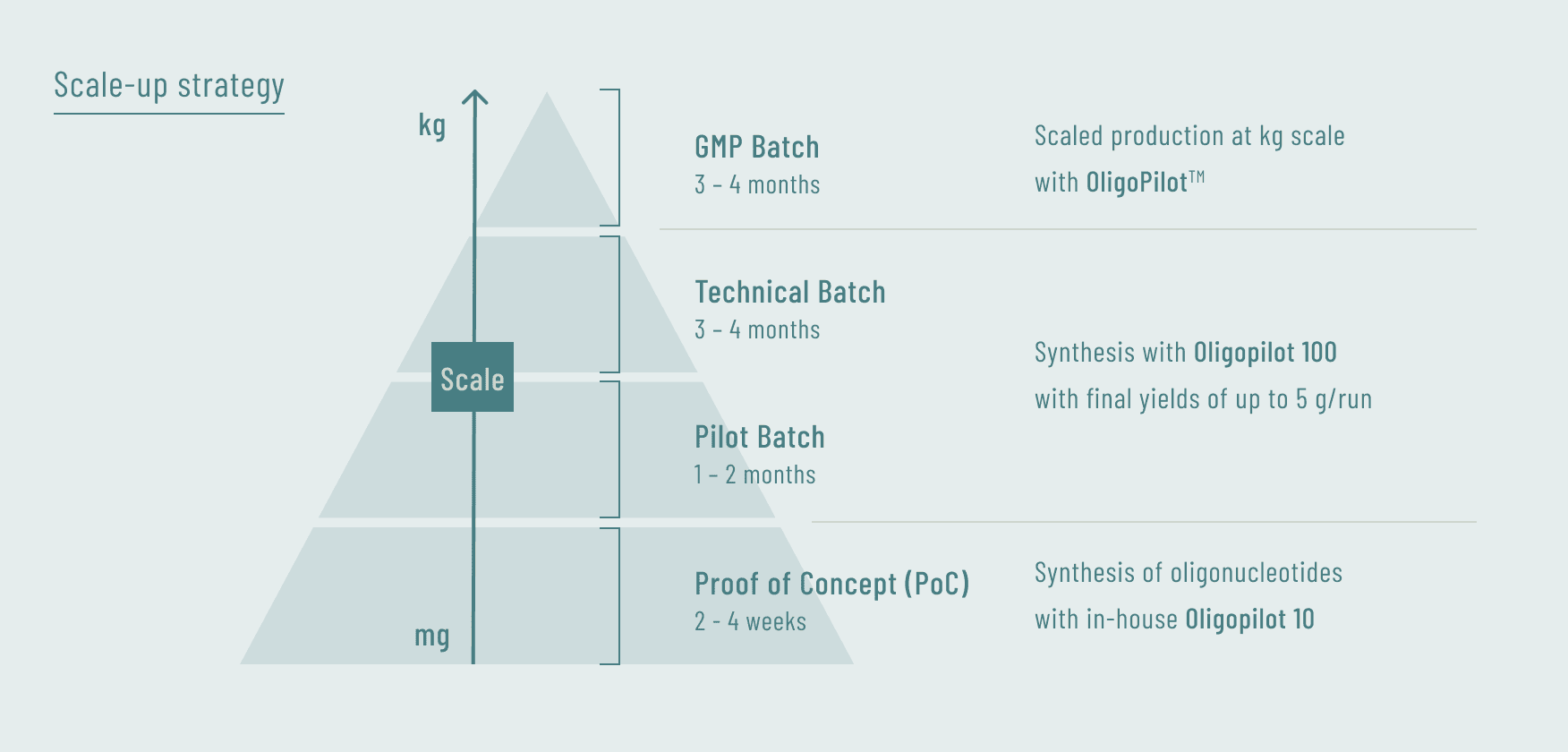
In process development, we have achieved seamless scale-up at each stage by equipping both our laboratory and production facilities with identical types of equipment.
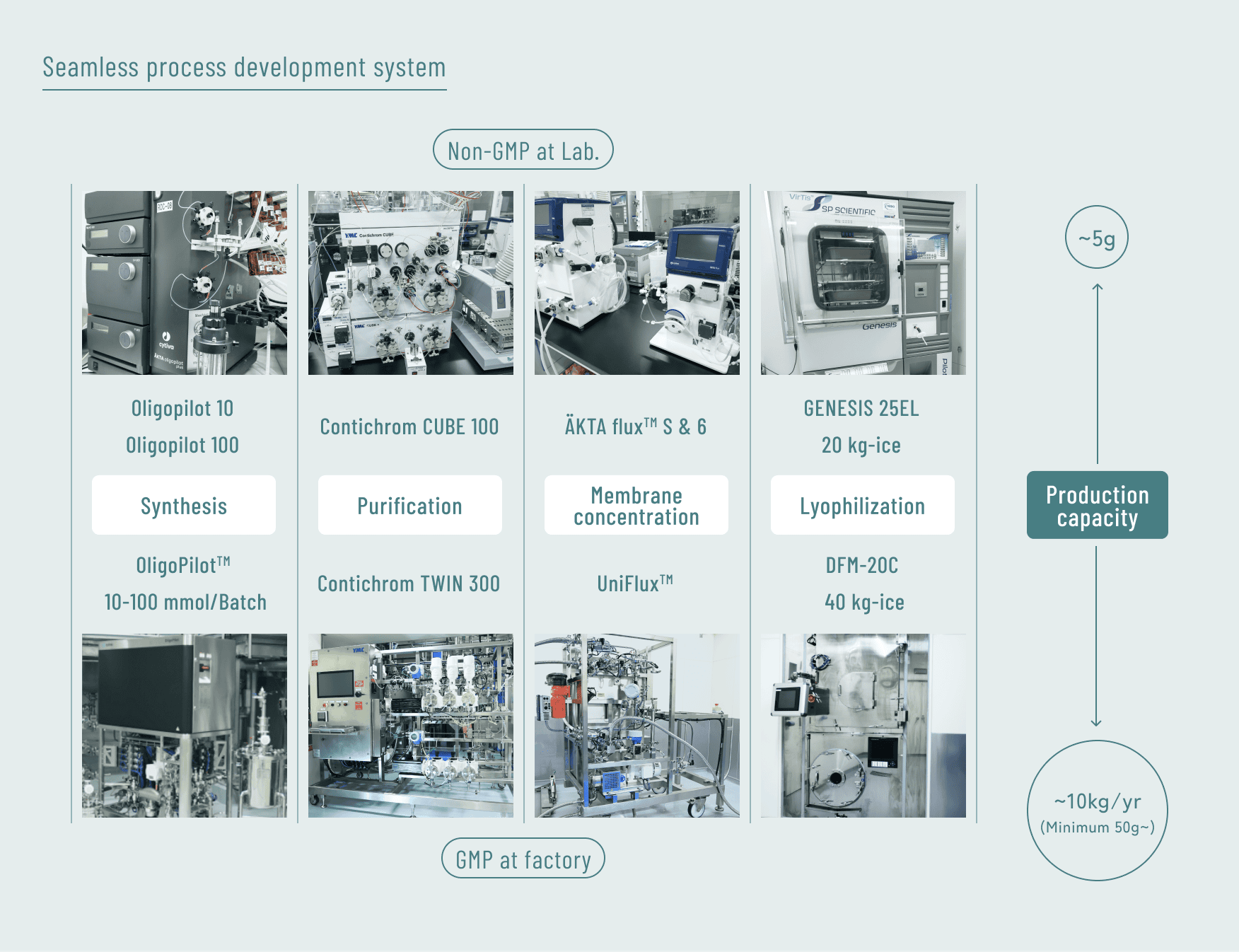
Main Services Offered

- Optimization of synthesis methods according to the specific sequence
- Quality and yield improvement through continuous purification technology
- Process development for scale-up
- Process development and manufacturing for novel modified nucleic acid monomers and conjugate molecules
Case Examples
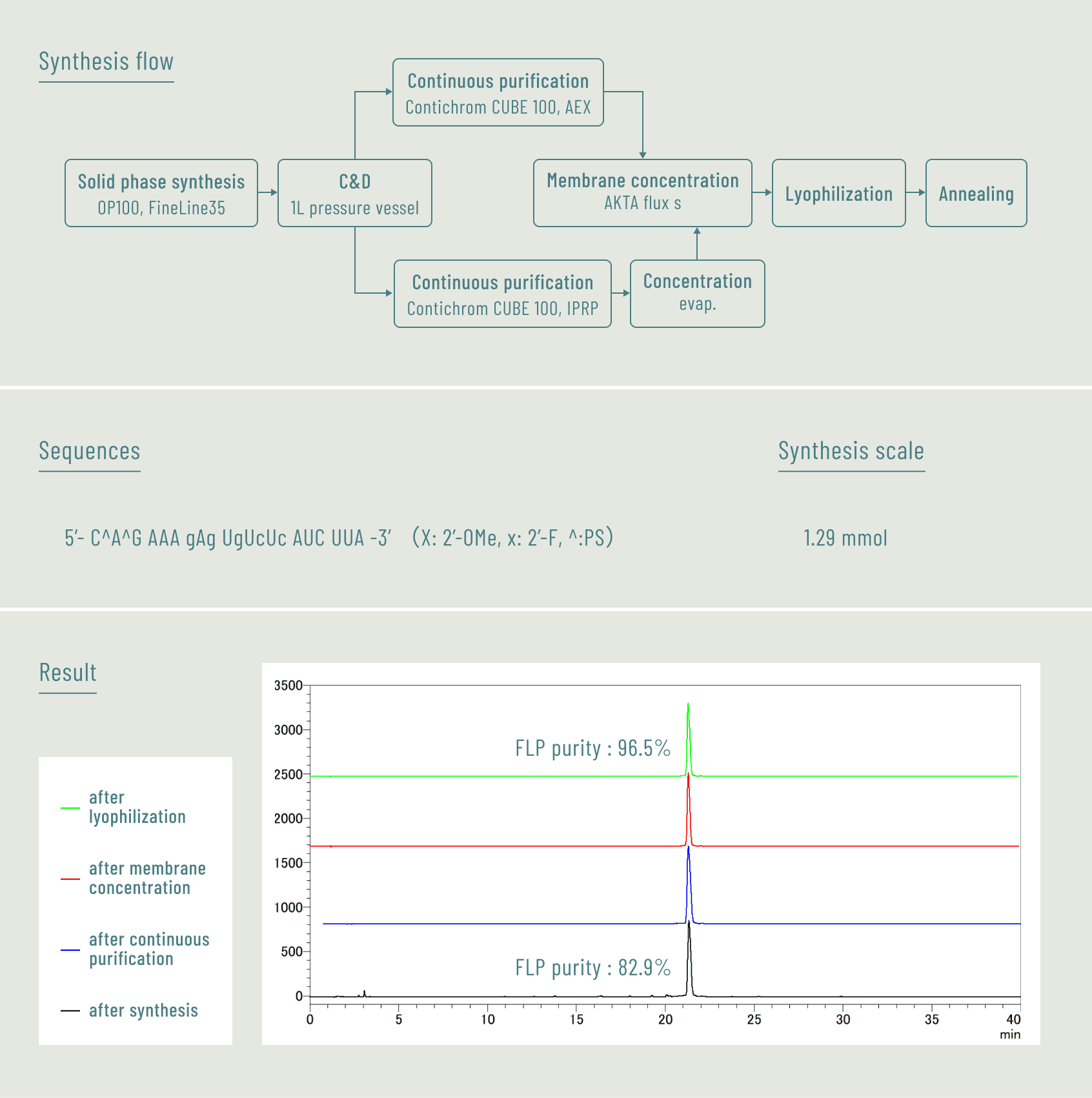
Process development and gram-scale laboratory sample synthesis
We will build optimal process in-house using only the sequence information of the nucleic acid oligomers.
Additionally, we will conduct sample synthesis at the gram-scale based on it.
Analytical Method Development
We have a wide range of state-of-the-art analytical equipment essential for oligonucleotide analysis, including low-adsorption UPLC, LC-Q-TOF/MS, sequence analysis software, and 2D-LC. This sophisticated instrumentation enables reliable data acquisition under stringent GMP controls. Leveraging our extensive expertise in analytical method development for small molecule APIs, we provide robust support in setting specifications and establishing analytical methods for oligonucleotides.
Data acquisition can be tailored to our customers' needs and all data, including analytical method development data, can be disclosed.
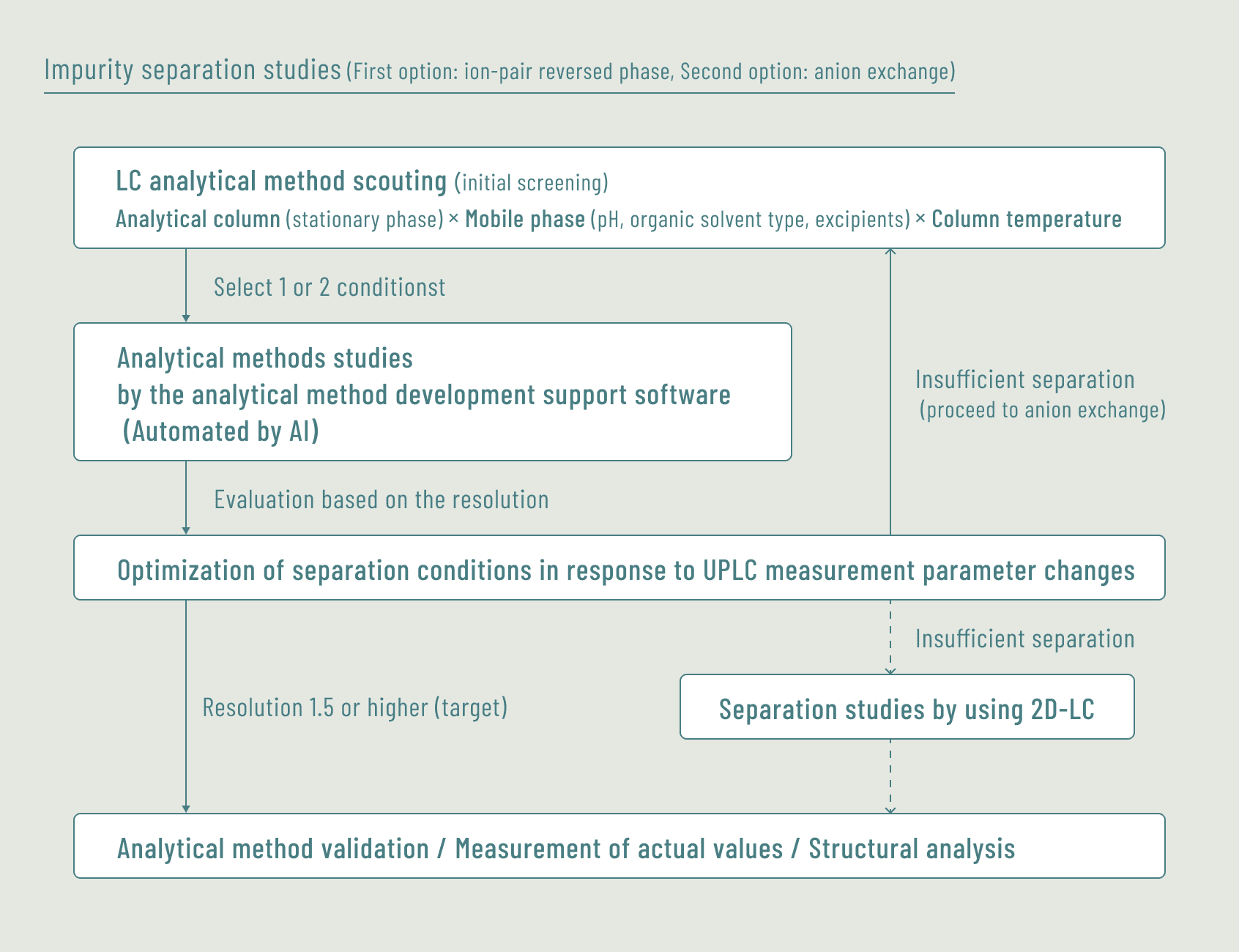
Utilizing various techniques to separate/analyze the impurities contained in oligonucleotides, we assist our customers in solving their impurity-related challenges.
Located adjacent to the Process Development Section, we can readily exchange information on impurity generation processes during the development stage and others. This collaboration allows us to propose practical analytical method development, utilizing the process information we gained.
As a result, we are able to provide a seamless development service from process development to analytical method development.
 LC-(Q)TOF/MS
LC-(Q)TOF/MS
 low-adsorption UPLC
low-adsorption UPLC
Main Services Offered
Analytical method scouting using the analytical method development support software (automated by AI)
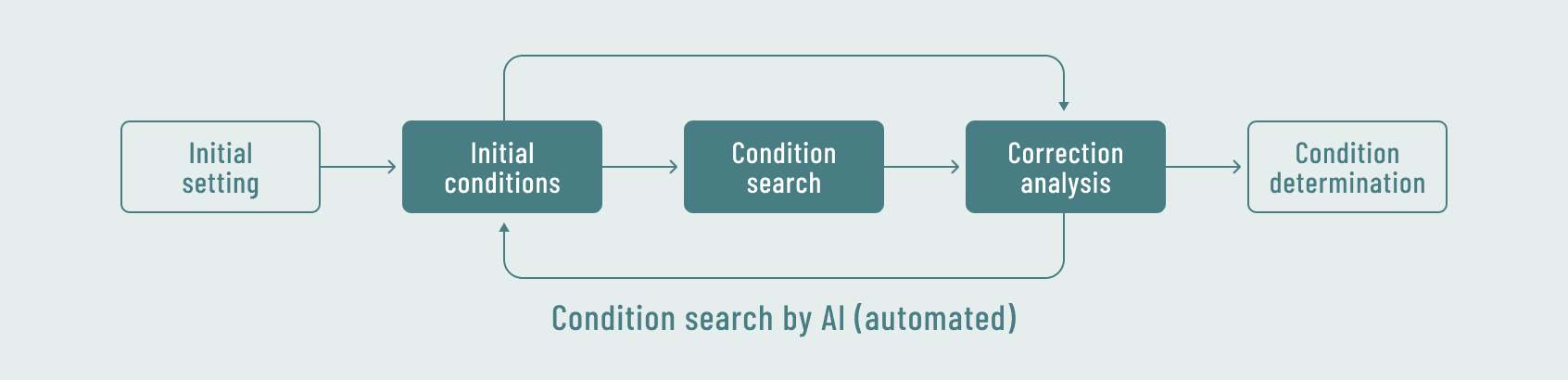
- Analytical method development (including method validation) and specification setting for identification testing, microbiological-related testing, physical property evaluation, and related substances for oligonucleotides.
- Separation of impurities contained in oligonucleotides by using 2D-LC system
- Structure analysis of impurities contained in oligonucleotides by using LC-Q-TOF/MS(including impurity profiling)
- Development and validation of highly sensitive analytical methods for mutagenic impurities(responding to ICH M7)
- Development and validation of analytical methods for residual solvents and elemental impurities(responding to ICH Q3C and Q3D)
- Data acquisition under the reliability assurance system based on GMP
Case Examples
Impurities profile preparation(for a major pharmaceutical company)
Separation and structural analysis of impurities contained in oligonucleotides by using 2D-LC(for a major pharmaceutical company)
Batch analysis of stability test results(for a major pharmaceutical company)
Quality evaluation of modified oligonucleotides
Development of various analytical methods and method validation for oligonucleotides and amides.
Inquiries
Please feel free to contact us even for small-scale production or small projects
Inquiries by Phone
[Available Hours] 8:30 – 17:00 on
weekdays (excluding Sat. Sun. and national holidays)
Contract Manufacturing





