CMC Solution Services
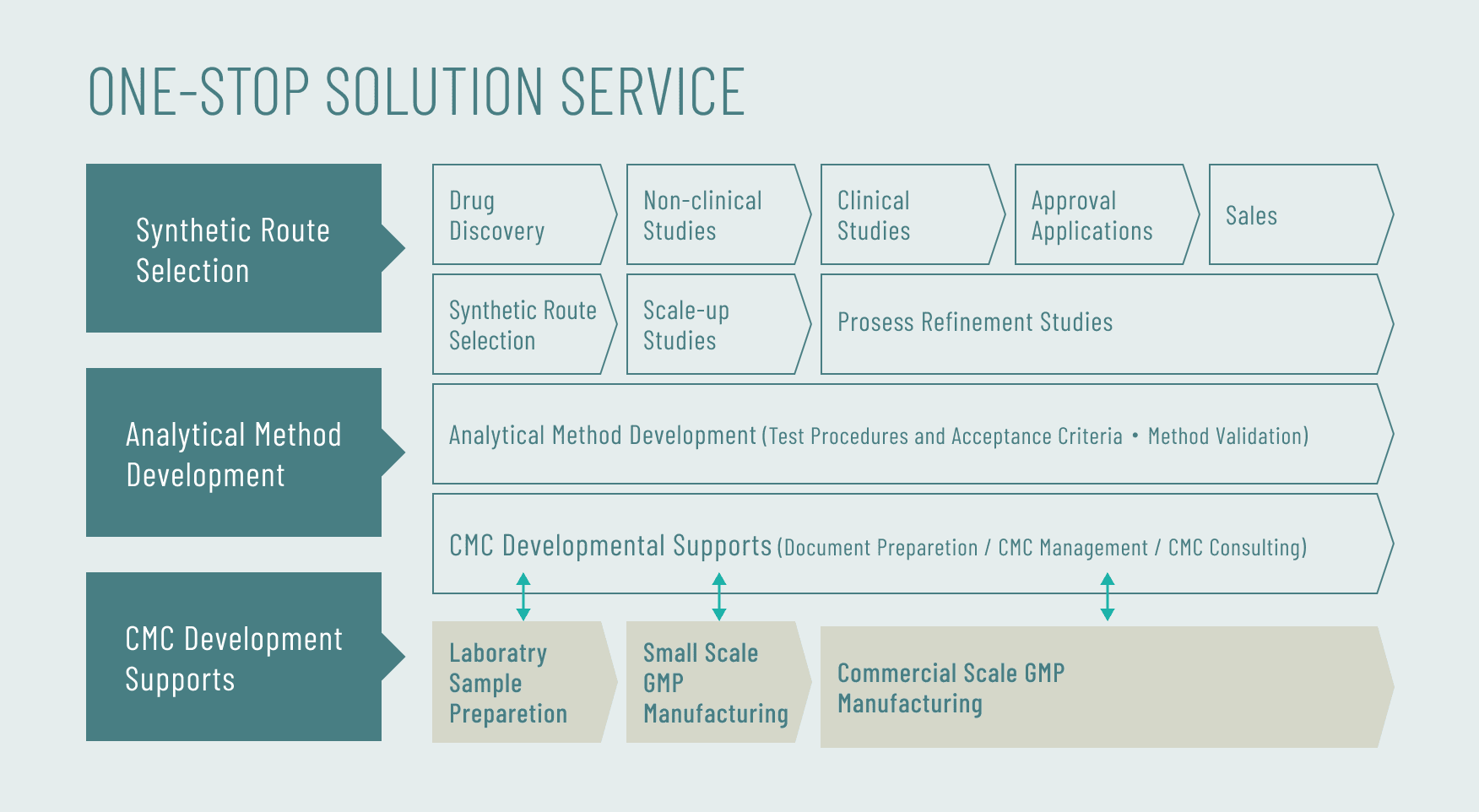
We provide CMC Solution Service, including Contract synthesis, Analytical test, Development of manufacturing process, Development pf analytical methods, and CMC development support, as One-Stop Service all through research and development stages.
Manufacturing Process Development

Comprehensive support for process development from sample synthesis of APIs/intermediates to synthesis method studies and scale-up studies
Capitalizing on the technologies and expertise acquired through our extensive experience in pharmaceutical contract manufacturing, we can meet the customers’ diverse needs by providing tailored manufacturing process and quality design for APIs.
Our process development experts provide seamlessly and swiftly CMC solution services that encompass all stages, from synthesis method evaluation, including route scouting, to API manufacturing.
Main Services Offered
- Contract synthesis of API/intermediate samples for various tests
- Synthetic route scouting based on safety of reactions, manufacturing costs, etc. (SELECT criteria)
- Synthesis of compounds and structure determination (impurities, metabolites, decomposition products, reference materials, etc.)
- Cause investigation for problems identified during actual manufacturing and proposal for improvement
Case Examples
Case ASynthesis route scouting for APIs/intermediates (for a major pharmaceutical company)
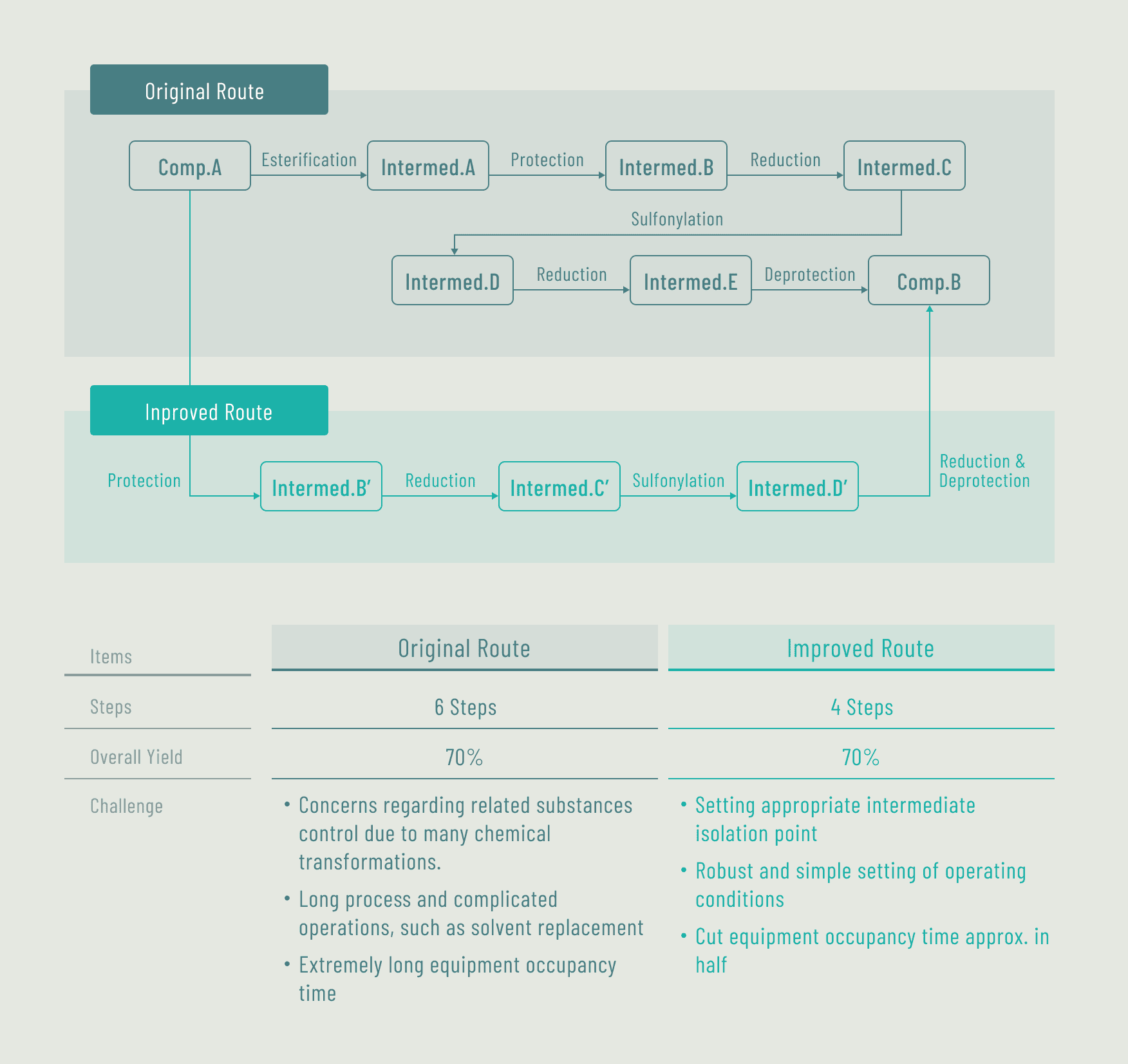
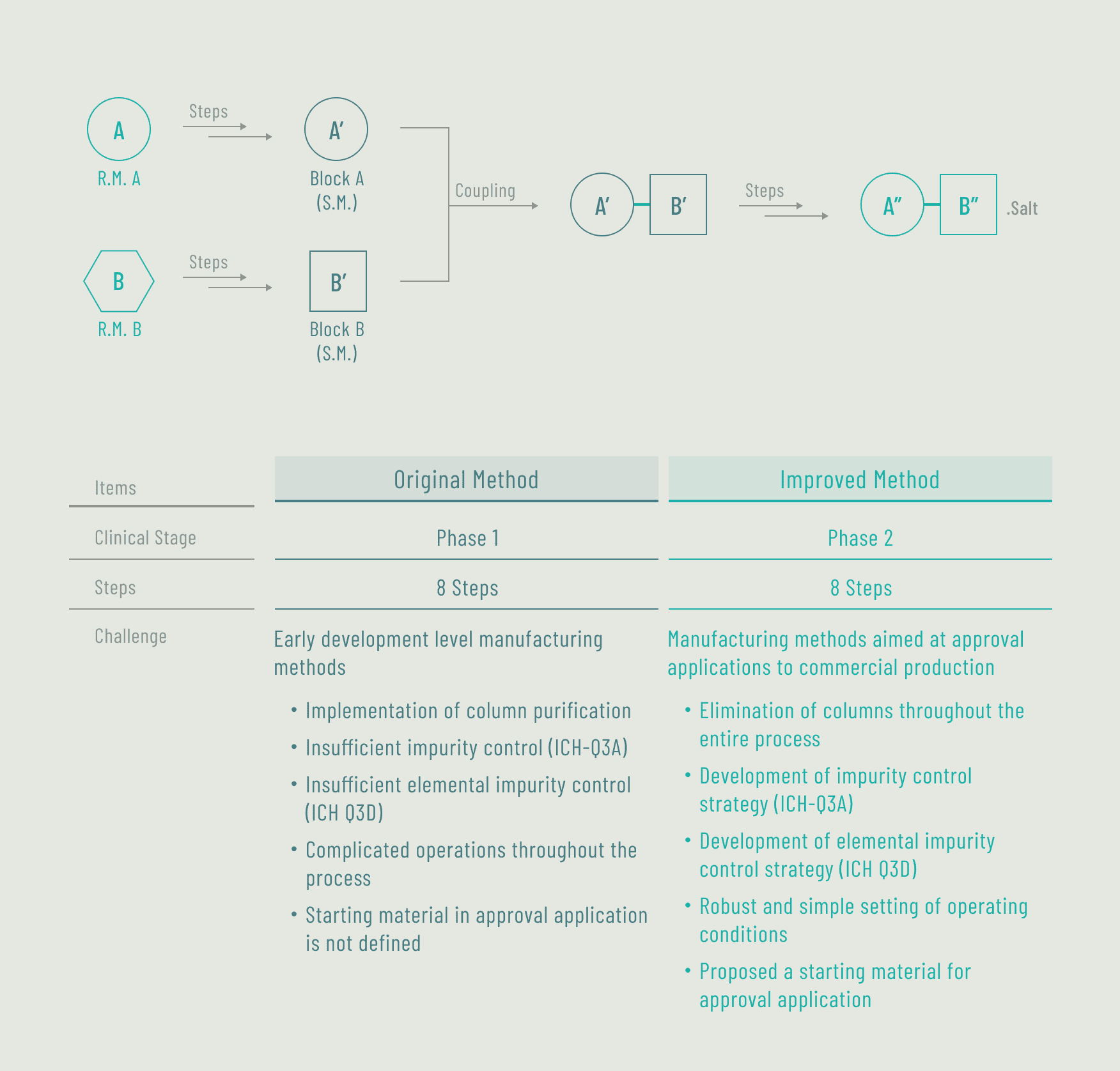
Case BFundamental improvement of API manufacturing method (for a bio-venture company)
Analytical Method Development

From rapid method development in the early development stage to robust method development for the later development stage and commercial production, a broad spectrum of customer needs can be met.
Our expert researchers, well-versed in analytical CMC development, support customers in solving quality-related challenges at every stage of drug development.
We are able to develop test methods for API quality evaluation, leveraging our deep understanding of impurity control strategies for drug substances and intermediates by working closely with manufacturing method development team. In addition, we can conduct various analytical tests in accordance with quality standards of pharmaceutical regulations and GMP.
Main Services Offered
- Analytical method scouting
- Development of test methods (including validation of analytical methods) and specifications setting for physicochemical tests, microbiological tests, physical property evaluations, and related substances, according to the development stage
- Development of highly sensitive analytical methods (including analytical method validation) for mutagenic impurities, etc
- Synthesis of compounds and structure determination (impurities, metabolites, decomposition products, reference standards, etc.)
- Development of elemental impurity analytical methods (including validation of analytical method) by using ICP-MS
Case Examples
Proposal of quality management strategy for APIs according to the development stage (for a major pharmaceutical company)
Analytical method development and method validation for investigational drug substances, intermediates, starting materials and in-process control tests (for a major pharmaceutical company and a drug discovery bio- venture company)
Setting of specifications and test methods for APIs according to the development stage (for a drug discovery bio-venture company)
Data acquisition for pharmaceutical manufacturing and marketing authorization application (for a major pharmaceutical company)
Preparation of IND application materials and investigator’s brochure (IB) (for a drug discovery bio-venture company)
Support for responding to inquiries from the regulatory authorities (for a major pharmaceutical company)
Establishment of purity test methods (related substances) (for a major pharmaceutical company)
Development of analytical methods for investigational drug substances (for a drug discovery bio-venture company)
Setting of specifications and testing methods (for a drug discovery bio-venture company)
CMC Development Support
CMC development support from the early stages of development through to new drug applications.
Our CMC development experts can meet the diverse customer needs from early development stage to approval application.
We provide a comprehensive long-term process management strategy – regulatory compliance, supply chain development, new drug applications, and commercial production – throughout the entire drug lifecycle as a One-Stop service by fully utilizing our manufacturing facilities.
Main Services Offered
- Proposal of CMC development strategies according to the drug development stage
- Acquisition of submission data for application using PAT (Process Analytical Technology)
- Proposals for validation protocol based on quality risk assessment (FMEA) regarding manufacturing process and API quality
- Impurities behavior tracing during the manufacturing process and supporting data acquisition for setting various quality standards (Spike & Purge study)
- Extraction of potential challenges that may arise in actual manufacturing and validation of reasonable acceptance range (PAR studies)
- Acquisition of Design Space using DoE and provision of supporting data for QbD application
- Preparation of application materials and documentation (e.g. IND, NDA and Drug Master File), and support for responding to inquiries from the regulatory authorities
- CMC development support, utilizing external resources (polymorphic screening, carcinogenic hazard assessment (impurities classification etc./ICH M7), label body synthesis, SDS authoring service, CMC consulting, intellectual property investigation, etc.), and project management
Case Examples
Case C Process parameter studies by using PAT tools as submission data for application (for a major pharmaceutical company)
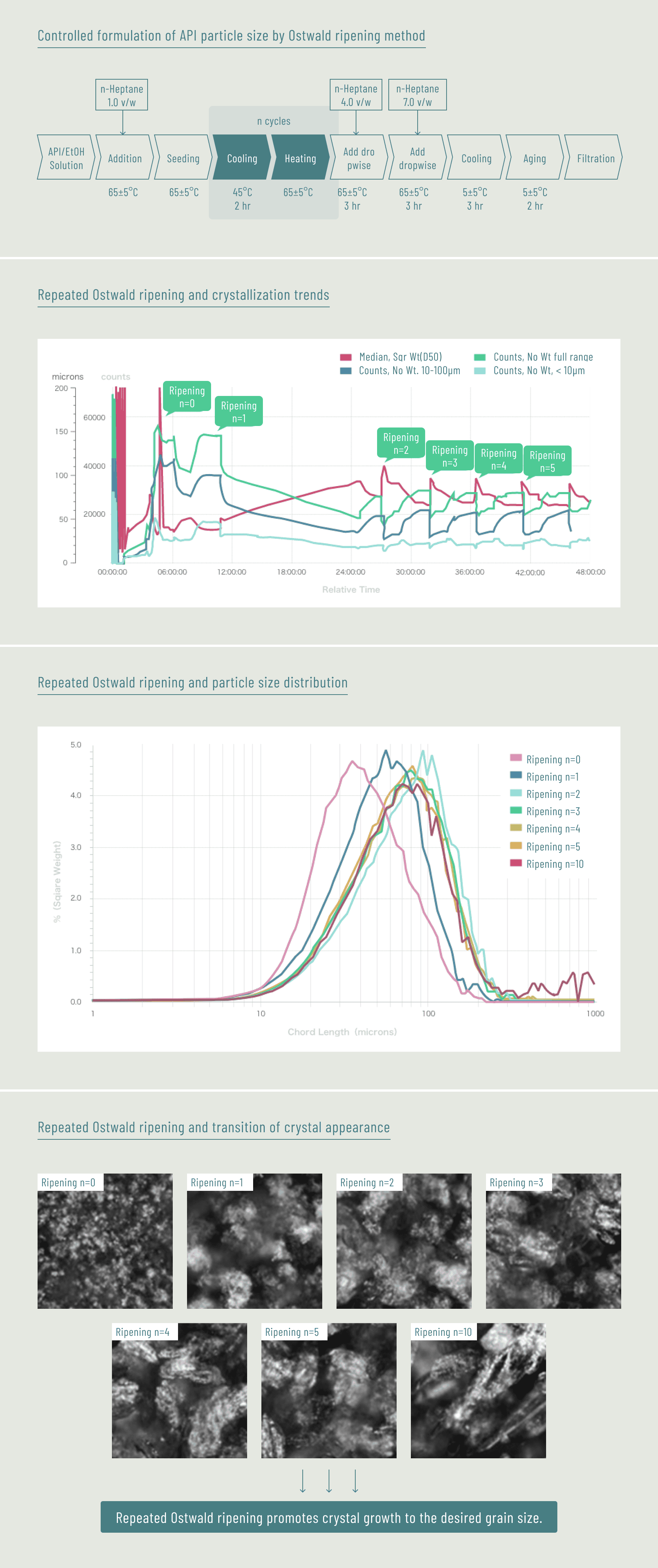
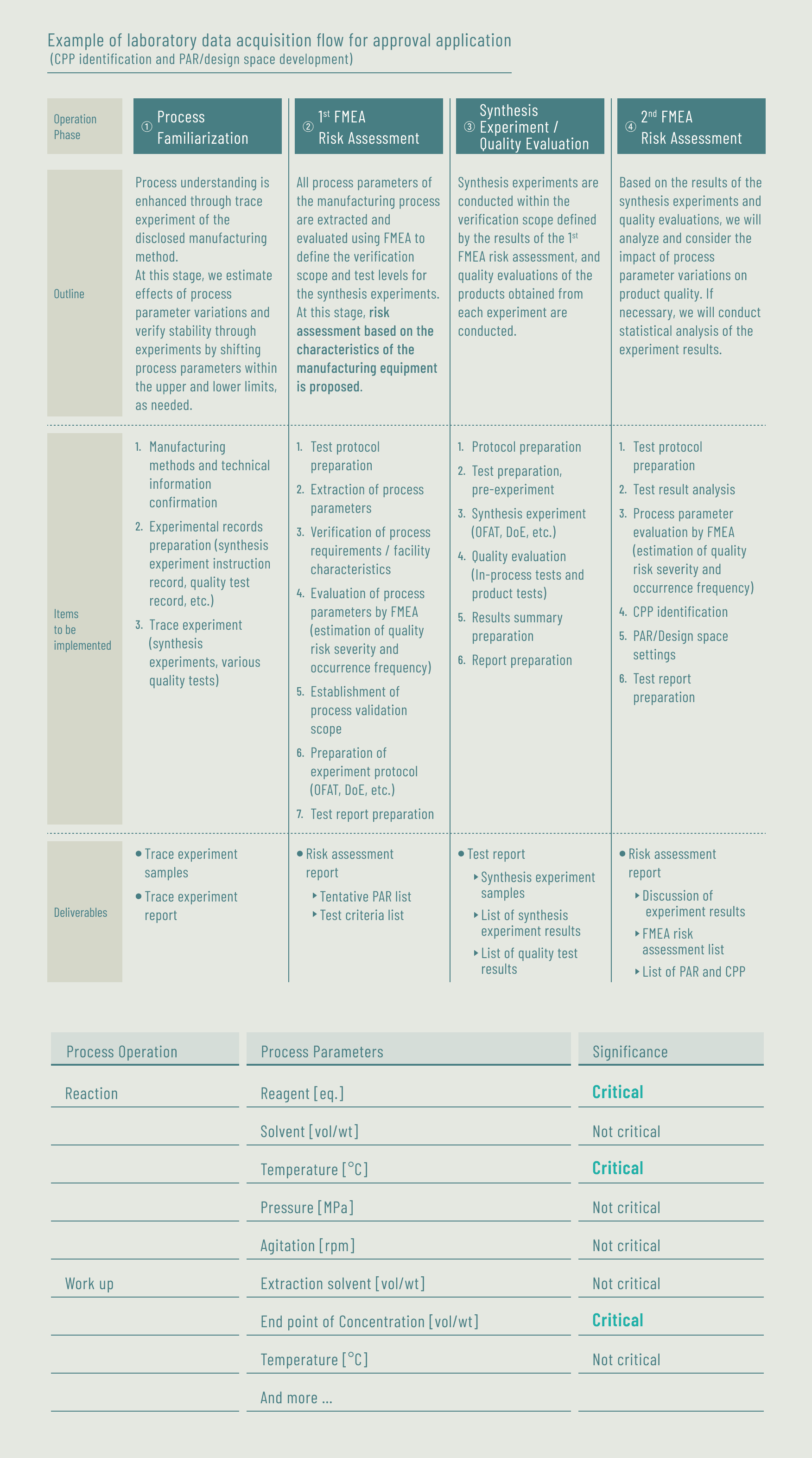
Case D Support for acquisition of laboratory data for approval application (for a major pharmaceutical company)
Inquiries
Please feel free to contact us even for small-scale production or small projects
Inquiries by Phone
[Available Hours] 8:30 – 17:00 on
weekdays (excluding Sat. Sun. and national holidays)
Contract Manufacturing





